How Pemflow / D.O.C. manage Extractables and Leachables Qualification of the in-process materials and primary packaging.
Août 31, 2022 | PEMFLOW

How Pemflow / D.O.C. manage Extractables and Leachables Qualification of the in-process materials and primary packaging.
An overview of a possible approach in the qualification from Extractables and Leachables perspective of the materials involved inside pharmaceutical production lines, from upstream to downstream processing, formulation and filling in the final forms.
Extractables and Leachables qualification are becoming in the last decade an important topic of discussion in the pharmaceutical scenario. International standard (ISO, ICH), European and US regulations were in the past mainly focusing on the primary packaging E/L qualification with no dedicated regulatory chapters on the in-process materials. With the increasing use of the Disposable technologies through the whole process stream the need of a basic harmonization in the E/L process assessment from the primary packaging up to the materials used in filling, downstream and upstream processing, is therefore strongly needed. The recent USP chapters on Extractables and Leachables assessment even if are still dedicating to the primary packaging are openly suggesting to extend the design of these studies also to all the other materials present into the specific process of course applying a correct risk-based approach to better identify the level and the criticality of the specific E/L assessment.
In addition to this an important difference is underlined between Extractables assessment performed for materials qualification studies compare to the Extractables (potential Leachables) assessment performed considering product and process parameters introducing the important concept of SIMULATING STUDY as an useful tool to predict and sometime, for the in-process materials, avoid a dedicated Leachables quantification.
Basically, the main differences between the two test configurations, as reported in USP <1663>, are as follow:
- Extractables Assessment for Material Characterization : one extracting solvent / one set of extraction conditions / one extraction technique /very aggressive extraction condition
- Extractables Assessment for Extractables-Leachables Correlation (Simulation Study)
- Multiple solvents or extracting media with varying extracting power based on the known extracting power of the drug product vehicle
- Multiple and complementary extraction techniques
- Extraction conditions that allow equilibrium to be achieved (worst case but not aggressive)
- Multiple Analytical Techniques/Systematic Process for Identification & Quantitation
- In practice the possible scenario following the USP <1660>, <1663> and <1664> chapters for in-process materials and primary packaging, can be summarized in the following three different step:
1. Material Screening and Qualification:
Characterization of a packaging system’s materials of construction to consider ingredients as probable extractables and potential Leachables. Such step facilitates the identification of materials that are suitable for use in packaging systems as well as the suitability for a glass type and container
- <660> Container: Glass
- <661> Plastic Packaging systems and their materials of construction
- <661.1> Plastic Materials of Construction
- <381> Elastomeric Closures for Injections
2. Simulating Extraction Study:
Worst Case (vs process/storage) controlled extraction study to determine extractables as potential Leachables.
- <661.2> Plastic Packaging Systems and their materials of construction
- <1663> Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery systems
- <1660> Evaluation of inner surface durability of Glass Containers (worst case approach)
3. Product Assessment:
During Stability (real time) to confirm potential leachables as Leachables
- <1664> Assessment of Leachables Associated with Pharmaceutical Packaging/Delivery Systems
- <1660> Evaluation of Inner Surface Durability of Glass Containers (Actual Use)
Simulating Study allows for consistent Extractables and Leachables correlation through the whole process stream (Upstream, Downstream, Formulation/Filling and Primary Packaging) between point 2. and 3. using a consistent and reliable approach.
As already underline, although the listed monograph are specific for the primary packaging, container closure and delivery systems, the principles and approaches to the qualification and definition of the chemical safety assessment can be extended to the overall materials and devices (Filter, Tubing, Connectors bags, …) present in the production line following a specific risk assessment.
Recent USP monograph still in draft like USP<665> and USP <1665>, recently implemented, are proposing an alternative route for the Extractables & Leachables assessment of the in-process components and materials based on the use of generic extractables data supplied by the article vendors (filters, tubing, bags, connectors or a complete single use system) obtained from a testing matrix/protocol well described within the BPOG protocol including a proposal of risk assessment that can help in the identification of the process area where the extractables data can be applied and help to identify the need of performing a Leachables assessment on the disposable components present within the process stream.
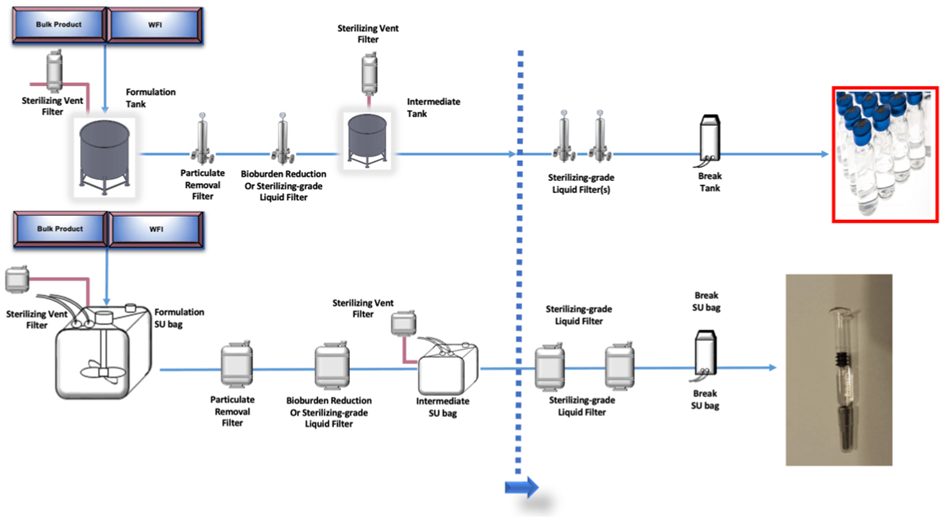
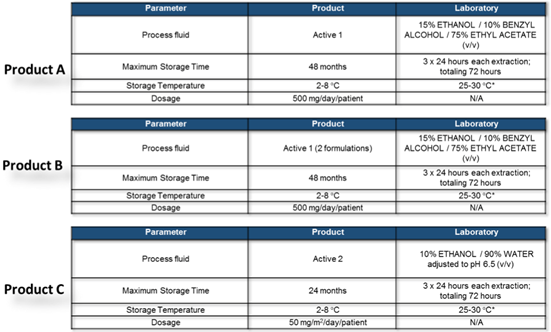
A practical example as a case study considering the Primary Packaging/ Delivery System (Final Forms Pre-filled Syringes & Plungers / Vials & stoppers) and transfer lines as in-process materials is reported below. A pharmaceutical company is producing three different final products (product A-B-C) collected into two main primary packaging forms. Production lines for product A and B foresee the use of stainless-steel systems (with the only exception for disposable filters, part of final tubings and 1 connector type). Production line for product C foresee disposable technologies (tanks, filters, tubing, connections).
The planned scope of the validation exercise is to cover the requirements of E/L on primary container only on product C and on primary container and in process material for product A and B following the indication coming from USP monographies <1660>, <1663> and <1664> including in the assessment a complete toxicological evaluation.
The design of the study foresees as FIRST STEP the identification of the different type of the component materials and suppliers of the primary packaging: primary packaging includes one type and one supplier of the pre-filled syringes and two different plungers (product A and Product B), while for product C a glass vials container (Type I glass) of different formats is used.
Product A & B are filled using transfer line stainless steel (disposable filters only) while for product C a full disposable line is used.
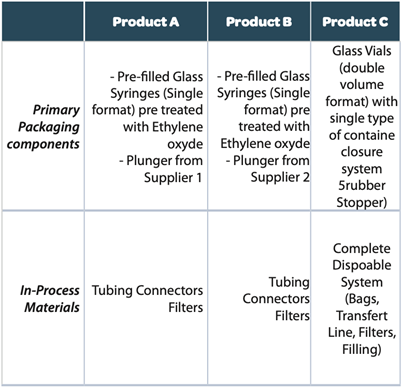
For primary packaging materials (vial/stopper/syringes and plungers) a total of 4 different suppliers were therefore included in the study design identifying 5 different types of materials to be included into the final assessment.
- The materials include: Borosilicate Glass Type I (Syringe Barrel and Vial)
- Elastomers (tip cap)
- Silicon oil/Silicone/ Dimethicone for lubrication coating (on plug only)
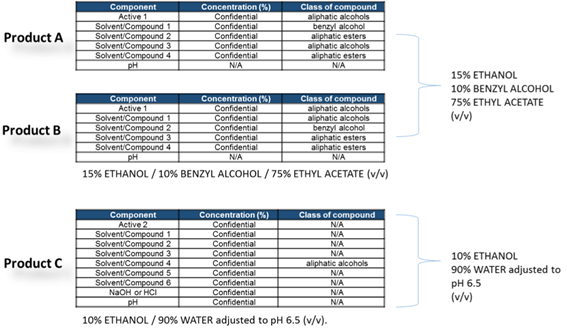
Once the list of the materials have been identified, the SECOND STEP of the study foresee the definition of the best extraction fluid to use to simulate the process/storage contact of the original drug product into the primary packaging or during the formulation and filling process step (filters, tubing, connectors etc..).
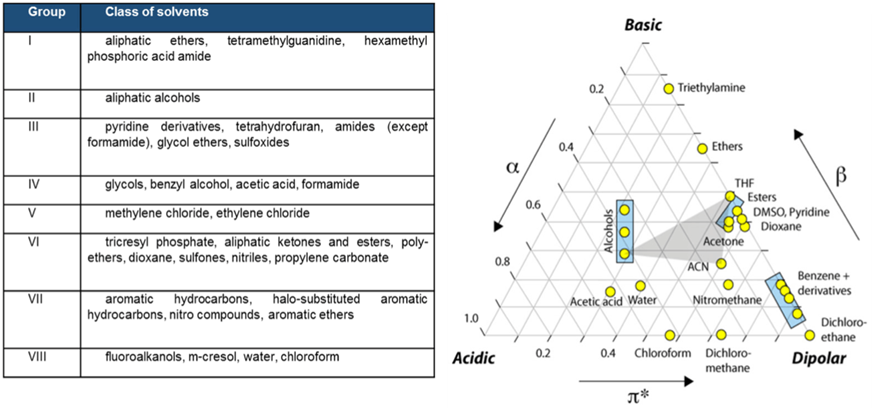
Main goal of this test phase is to create a simulation solvent(s), that can be a pure solvent or a mixture of different solvents, that is able to create extraction conditions worst case compare to the original drug product but NOT in an exaggerated way like the extraction conditions normally followed for material qualification as reported into USP <660>, <661> and <381> : this solvent(s) should be selected in order to mimic the leaching power of the original drug product following, in this case, the modeling rules suggested by Snyder’s classification on functional groups and solvents polarity.
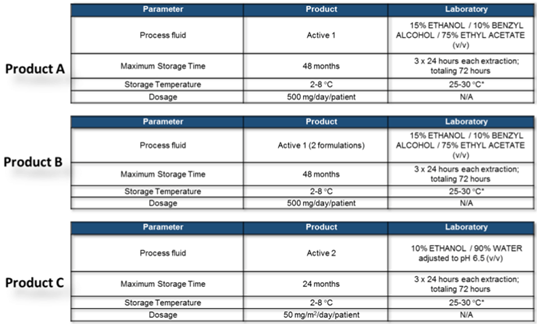
Due to very similar composition of product A and product B the simulating solvent mixture obtained from the modeling exercise is identical for both products, while for product C the different product composition and chemical-physical parameters are suggesting the use of a different mixture including water and pH control.
Once the simulating solvents are identified the second step, to complete the approach of SIMULATING STUDY is to consider the Process (in-process materials) and Storage/Stability (Primary Packaging) conditions.
The solvent classification is based on their polarity and their selectivity or relative ability to engage in hydrogen bonding or dipole interactions. According to these factors, common solvents can be classified into eight groups as follows :
For primary packaging the process condition can be easily identified considering the storage condition : to generate the «reasonable» worst case a controlled extraction study can foresee the use of higher temperature that can mimic worst case conditions and that could be reached in OOS process parameters (ex. normal storage condition between 2-8°C an extraction temperature close to room temperature can be applied (25-30°C) avoiding in such way a very aggressive conditions normally used in the material qualification studies (e.g. 50° – 70°C).
The efficacy of the extraction step can be easily verified splitting the extractions on the same material in several extraction repetition (35) in order to confirm the depletion of the extractables obtained from each extraction step.
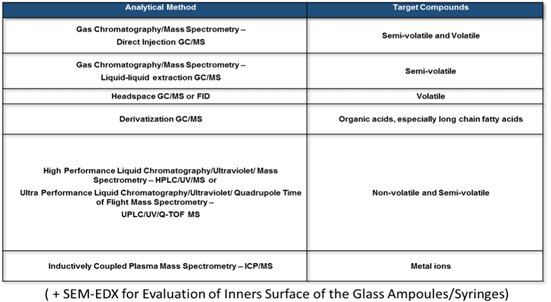
Analytical Methods once calculated the AET limit considering the SCT of 1.5μg/day specific for parenteral drug product, will be chosen, as suggested by the USP <1663> chapter, in order to cover the requirements of methods for SCOUTING, DISCOVERY IDENTIFICATION and QUANTITATION of the overall non-volatile, semi-volatile and volatile compounds.
RESULTS on Primary Packaging/Delivery System
- 14 compounds (9 organics and 5 inorganics) above the AET, identified and quantified (semi-quantitative analysis)
- No “unknown” compounds detected
- No delamination phenomena detected on glass inner surfaces (SEM Analysis)
- Toxicological Assessment performed on the 14 compounds with specific PDE calculation for each compound.
- Leachables Study on-going focusing on the 14 compounds detected and considering real stability time with intermediate sampling points (ex 48 months: 3-6-12-24-48 ).
RESULTS on In-Process materials
(Tubing, Filters, Connectors, Single Use disposable System):
- Studies will be done applying the same Simulation Study approach (modeling the products with multiple solvents) and identical analytical techniques.
- 5 compounds identified (4 organic s 1 inorganic) 4 of them already present in the primary packaging scenario.
- No “unknown” compounds detected.
- Toxicological assessment performed on the single compound identified from the materials in process.
- No concern from toxicological point of view and no Leachables assessment required on the in-process Materials analyzed.
These information will complete the validation matrix on materials qualification (primary packaging and in-process materials) that will be an important step of the overall assessment that should include: Biological Safety Tests, Materials Qualification, Simulation Studies, Toxicological Assessment and Leachables Assessment during stability scenario as reported by USP monographs.
Conclusion
USP Chapters <1663>, <1664>, <1660> on Extractables/Leachables assessment and Inner Surface Durability of Glass Containers are focusing on the needs of perform such type of tests considering Chemical/Physical Parameters of actual drug product as well as the specific Process (in- process materials) and Storage (primary packaging) parameters. This is the most completed and safe evaluation that can be performed in order to complete a “chemical-safety assessment” of the material in process and in primary packaging.
- Simulation Approach allows to generate extractables data (“potential Leachables”) that can be used for an Extractable/Leachables correlation
- Simulation Approach can be applied to the whole process stream: in-process materials and final forms.
- The Simulation Approach is Product & Process Specific and can be applied to the specific Device (in-process material or primary packaging) following a risk-based approach.
- If the extraction conditions in a controlled and simulated extraction study is able to mimic the“leaching power”of the processed and packaged drug product, the packaged drug product’s conditions of clinical use (including distribution and storage), then the extractables profile of the production line and packaging system will mirror the final Leachables profile of the packaged drug product.
Antonio LEGNANI – PEMFLOW
www.a3p.org/how-pemflow-d-o-c-manage-extractables-and-leachables-qualification
La Vague – Nr74 – July 2022







